Allercept™ Testing
The most sensitive and specific serum allergy assessment in the industry.
Designed for cats, dogs, and horses that have been diagnosed with atopic dermatitis.

Sensitive
Patented technology detects as little as 10 picograms of allergen-specific IgE.
Accurate
High-affinity IgE receptor safeguards against misidentifying allergy sensitivities based on IgG detection.
Comprehensive
Universal panel detects sensitivities to 83 environmental allergens and 24 food allergens in dogs and cats, 91 environmental allergens in horses.
Convenient and Fast
Testing kit provides everything needed to submit a serum sample. Results within two business days.
Customized
Recommendations based on assessment results, patient history, cross-reactivity of allergens, and geographic location.
ALLERCEPT™ offers long-term relief without the long-term adverse effects of lifelong drug therapy.
By addressing the source and not just the signs, immunotherapy is the only treatment option that can potentially relieve both the clinical symptoms and prevent disease progression – all with minimal adverse effects for another quality-of-life win.
Concomitant medications to control pruritus can be safely used along with immunotherapy to provide immediate itch-control, while building the foundation for long-term relief.
Highly Successful Treatment
Allergen-specific immunotherapy has proven efficacy in dogs, cats and horses with response rates between 50-80%.1
Allercept™ testing measures the level of IgE antibodies to individual allergens within the blood.
Designed for dogs, cats, and horses that have already undergone an allergy workup and have been diagnosed with atopic dermatitis.
Technical Details and Downloads
Overview
Allercept™ Immunotherapy Treatments
The exact mechanisms of allergen-specific immunotherapy (ASIT) are not fully understood, but it is clear that the treatment influences multiple immune cells and processes.
Dendritic cells — also known as antigen-presenting cells — are located throughout the skin, gastrointestinal tract, and mucosal surfaces. Their role is to capture allergen molecules and migrate to local lymph nodes, where they instruct the immune system on how to respond.
ASIT prompts dendritic cells to promote the production of regulatory T lymphocytes and other molecules that help down-regulate the immune response.
Overall, immunotherapy shifts the immune reaction from an abnormal Th2/IgE-dominant response to a more balanced Th1/IgG-mediated response by down-regulating IgE and up-regulating IgG.
The process begins at the point of care with a simple blood draw that is submitted to our labratory. Our patented Allercept™ allergen-specific IgE test identifies important allergens. Patient recommendations are based on Allercept test results, clinical history of the allergic animal, and geographic region.
While every animal responds differently, most patients respond positively to Allercept Immunotherapy Treatments, with most patients responding positively within 4-6 months, and best results reached 10-12 months.
Allergen-specific immunotherapy (ASIT) is successful in 50-80% of dogs, cats and horses. In a retrospective study of dogs, best success was demonstrated inpatients that receive regular, quarterly check-ups, strict compliance to administration of the serum and no use of systemic corticosteroids1.
ASIT has been effectively used since 1941, is generally regarded as safe and has been shown to reduce the use of concomitant medications in dogs by 87%2.
Highest Standards in Quality Control Protocols
- Immunotherapy is manufactured in a clean room environment with the added protection of extensive sterile gowning and containment hoods to ensure the highest quality product.
- Each patient therapy manufacturing record is triple checked by the QA team, providing additional assurance that each patient is receiving the exact product ordered.
- The immunotherapy manufacturing process utilizes personnel that are educated and extensively trained to perform their duties.
- The product is manufactured to ensure complete lot traceability for each treatment set.
Technology
Allercept™ offers the most sensitive and specific serum allergy assessment in the industry1,3. Its patented technology can detect as little as 10 picograms of allergen-specific IgE to identify the source(s) of allergic reaction. And unlike many other serum IgE tests that can misidentify allergy sensitization based on the detection of allergen-specific IgG, Allercept’s high-affinity IgE receptor safeguards against such false positives.
Allercept™ Therapy Shots
Extracts of the identified allergens are mixed together and administered to the patient by subcutaneous injection, starting with very tiny doses and gradually building up to very large doses. This treatment was formerly called “desensitization” or “hyposensitization” and is often referred to in lay terms as “allergy shots.” For those patients that don’t respond to injections, switching to drops was reported to be effective in 50% of these dogs.4
Allercept™ Therapy Drops
Allercept Therapy Drops is a serum given under the tongue twice daily.
The mucosal area under the tongue is a privileged immunological site with unique characteristics consisting of local immune cells. This allows for differentiation of pathogens from harmless antigens, muting of immediate and delayed allergic response, and tolerance of mucosally delivered allergens.
Sublingual immunotherapy allows specific antigens placed under the tongue to induce immunologic tolerance. Multiple mechanisms are involved and include the production of anti-inflammatory cytokines such as IL-10 and the induction of regulatory T-cells.
The mucosal area under the tongue is a privileged immunological site with unique characteristics. It consists of:
A physical barrier (mucosa)
Local immune cells
— High concentration of dendritic cells and T-cells
— Low concentration of mast cells, basophils and eosinophils
This allows for:
- Differentiation of pathogens from harmless antigens
- Muting of immediate and delayed allergic responses
- Tolerance of mucosally-delivered allergens
Safety and Efficacy
Allergen Specific Immunotherapy (ASIT): in a systemic review by the International Task Force on Canine Atopic Dermatitis, ASIT was found to be safe and effective.5
For horses: ASIT is generally well tolerated in horses, with extremely rare systemic reactions.6
Our proprietary technology for sublingual administration was developed as a veterinary-specific variation based on a unique, time-tested and highly efficacious human protocol. In efficacy trials with hundreds of atopic dogs treated by veterinary dermatologists in varying geographic areas of the U.S. over the past 3 years, approximately 60% of dogs demonstrated substantial improvement in their clinical signs. Approximately 50% of dogs that showed no improvement or could not tolerate allergy shots, showed improvement when treated with allergy drops.
Allercept Therapy Drops can even be used safely in dogs that have experienced a prior anaphylactic reaction to allergy shots. If the patient has a history of prior anaphylactic reaction to allergy shots, please contact Antech’s Specialty Products Technical Support at 800-464-3752, option 5.
Downloads
Contact us at 800-464-3752, Option 5 or [email protected] to request copies of any of the materials below:
Sources
- Fennis EE, van Damme CM, Schlotter YM, et al. Efficacy of subcutaneous allergen immunotherapy in atopic dogs: a retrospective study of 664 cases. Vet Dermatol. 2022;33(4):321–e75.
- Ramió-Lluch L, Brazís P, Ferrer L, Puigdemont A. Allergen-specific immunotherapy in dogs with atopic dermatitis: is owner compliance the main success-limiting factor? Vet Rec. 2020;187(12):.
- Stedman K, Lee K, Hunter S, Rivoire B, McCall C, Wassom D. Measurement of canine IgE using the alpha chain of the human high affinity IgE receptor. Vet Immunol Immunopathol. 2001 Feb 10;78(3-4):349-55. doi: 10.1016/s0165-2427(01)00242-2. PMID: 11292535.
- Marsella R, Ahrens K. Investigations on the effects of sublingual immunotherapy on clinical signs and immunological parameters using a canine model of atopic dermatitis: a double-blinded, randomized, controlled study(abstract). Vet Dermatol 2012;23(Suppl 1):66.47
- Olivry, T, DeBoer, DJ, Favrot, C, Jackson, HA, Mueller, RS, Nuttall, T, Prélaud, P; International Task Force on Canine Atopic Dermatitis. Treatment of canine atopic dermatitis: 2010 clinical practice guidelines from the International Task Force on Canine Atopic Dermatitis. Veterinary Dermatol. 2010 Jun;21(3):233-48.
- Marsella R, White S, Fadok VA, Wilson D, Mueller R, Outerbridge C, et al. Equine allergic skin diseases: Clinical consensus guidelines of the World Association for Veterinary Dermatology. Vet Dermatol. 2023; 34: 175–208.
We’re here for whatever you need — when you need it.
Product usage questions, case discussions, and everything in between.
Antech™ Support
- Monday – Friday, 6:00 a.m. to 5:00 p.m. MST
- 800-464-3752 (U.S.), Option 5
- Leave us a voicemail after business hours.
Consider these Antech™ solutions for the right healthcare, at the right time.
-
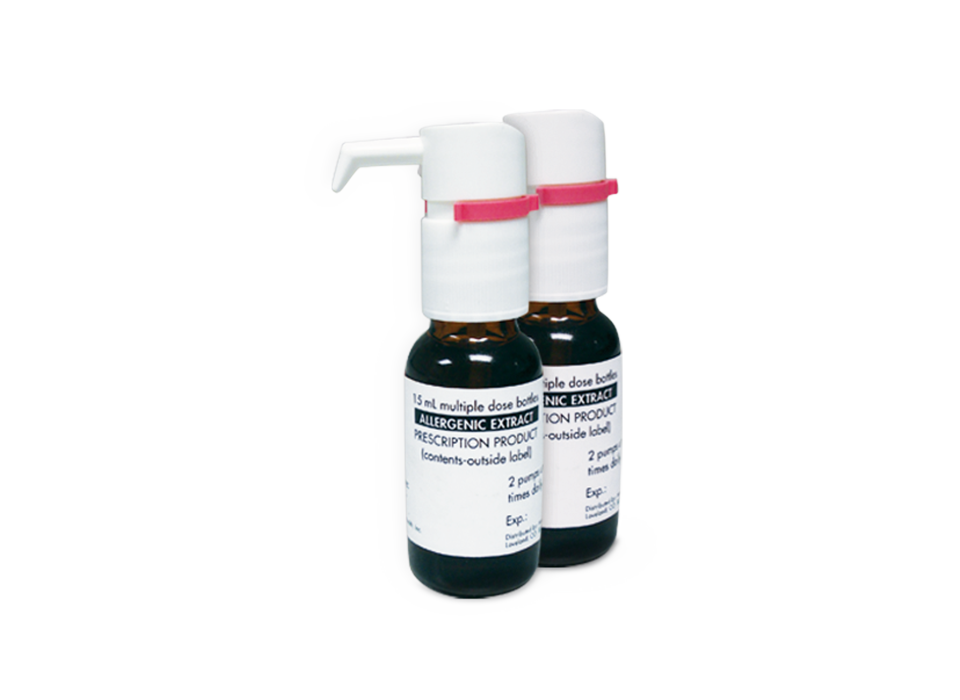
Allercept™ Therapy Drops
Long-term immunotherapy-based allergy relief for dogs, cats, and horses, delivered under the tongue.
-
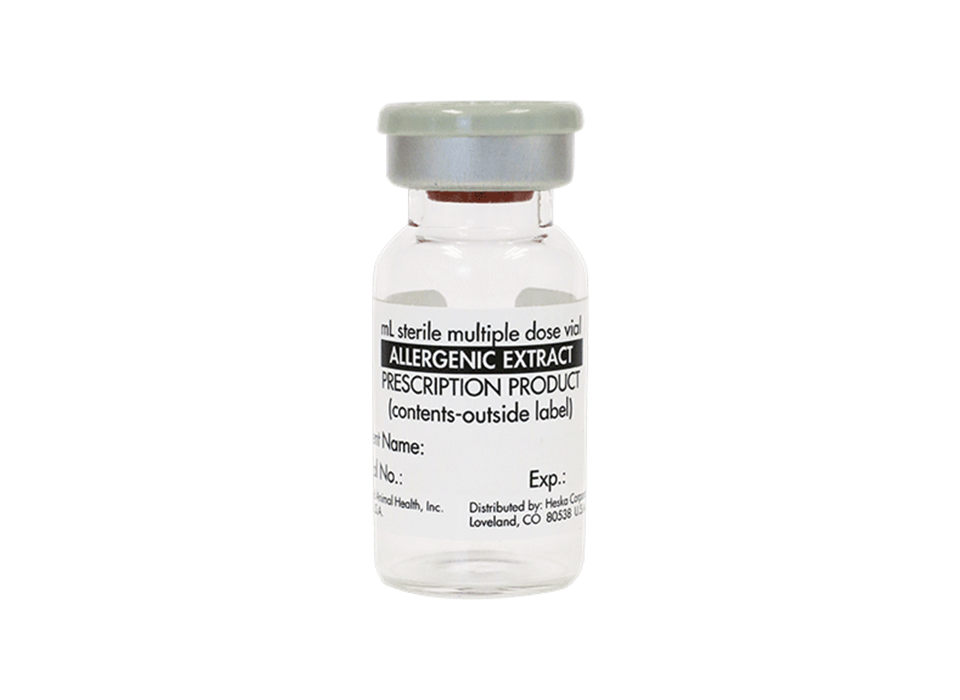
Allercept™ Therapy Shots
Long-term immunotherapy-based allergy relief for dogs, cats, and horses.
Have questions about Allercept™ Testing?
Complete the form and an Antech representative will reach out to you.
