Accurate Diagnostics, Smarter Prescribing: The Twin Engines of Antimicrobial Stewardship
By Meera Surendran Nair, DVM, MS, PhD, DACVM
Director, Infectious Disease at Antech
Antimicrobial resistance is a growing global problem. Not only have the resistance patterns of some organisms changed over time, but many bacteria now carry at least some level of resistance to antibiotics and antimicrobials.
This is a serious issue, not only for animals, but also for humans. And if we clinicians are not using the correct guidelines to treat organisms — dosing the right antibiotics for the right organisms at the right time — then we are unintentionally contributing to a worsening problem.
Accurate culture and antibiotic susceptibility testing (AST) is the cornerstone of antimicrobial stewardship. To understand the critical role of diagnostics in antimicrobial stewardship efforts, we first must recognize how antibiotic susceptibility testing (AST) and clinical breakpoints function.
A refresher on antibiotic susceptibility testing (AST) and clinical breakpoints
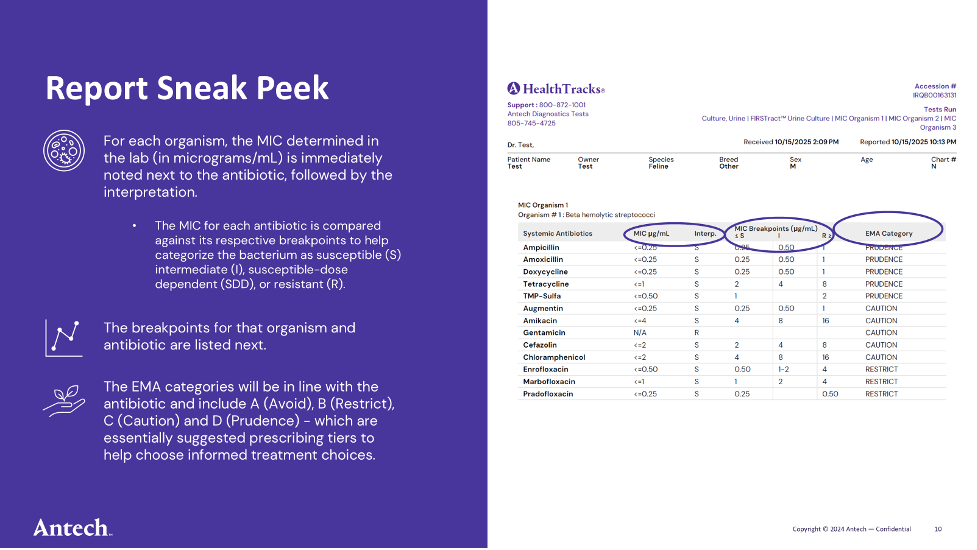
Clinical breakpoints are the cut-off values we use to decide whether an antibiotic is likely to work or not. When a lab grows bacteria from a patient and measures the MIC—the lowest concentration of antibiotic that stops growth—we compare that number to the established breakpoints.
If the MIC is below the susceptible breakpoint, the antibiotic is likely to be effective. If the MIC is above the resistant breakpoint, the antibiotic is unlikely to work.
Breakpoints are built from large datasets and help translate a lab measurement into a practical “yes,” “maybe,” or “no” for treatment.
Both the Clinical and Laboratory Standards Institute (CLSI) and the European Committee on Antimicrobial Susceptibility Testing (EUCAST) develop and regularly review clinical breakpoint standards used in AST, including standards specifically for use in veterinary medicine. Clinical breakpoints are revised periodically to ensure the levels are optimally set using newly available data (e.g., evidence of increased resistance dynamics in a wild-type population, availability of pharmacokinetic and pharmacodynamic data for a drug) to align with the clinical outcomes.
The most recent review of antibiotic clinical breakpoints prompted several major changes to ensure optimal prescribing:
- Breakpoints were lowered for some drugs. Meaning the reported resistance for some bug-drug combinations increased.
- Some bug-drug combinations now have veterinary-specific breakpoints for certain bacteria species. Human breakpoints were used previously.
- There are now different breakpoint adjustments for different body systems (e.g., urine vs. plasma). Different breakpoints will be used for urine submissions vs. tissue for some bug-drug combinations.
- For some drugs, a breakpoint is now available for different dosages. These are listed under a new interpretation category termed “susceptible dose dependent (SDD).”
These changes influence report interpretation, antimicrobial stewardship metrics, and downstream treatment decisions, so it’s essential to be aware of the updates when you are determining the treatment. Moreover, the AST updates are also a step change for diagnostic labs with refined testing workflows seeking to deliver more clinically actionable results.
Using gold standard diagnostics for quantitative susceptibility testing
Broth microdilution remains the reference standard for determining minimum inhibitory concentrations (MICs). Our laboratory employs an automated incubation and plate-reading platform that standardizes test conditions, reduces manual variability, and produces consistent, quantitative MIC data. These standardized results allow breakpoint-based interpretations that are more directly actionable for clinical decision-making.
At Antech, we prioritize diagnostic workflows that enable precise organism identification and quantitative susceptibility results, supporting clinicians in selecting targeted therapy and minimizing unnecessary antimicrobial exposure. We integrate matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) into our identification workflow to provide rapid, high-throughput species-level identification with analytical accuracy. For obligate anaerobes, we use controlled anaerobic culture environments to ensure appropriate recovery and characterization. Combining robust identification with rigorous culture techniques strengthens the link between organism identification and susceptibility testing.
AST panels curated for veterinary species
Our antimicrobial susceptibility testing panels are curated for clinical relevance rather than sourced as generic, one-size-fits-all products. Each panel is developed with consideration of host species, common site-specific pathogens, and antimicrobials used in practice. We maintain species-specific panels for canine, feline, and equine patients, and provide tailored profiles for large animals, primates, exotics, and poultry. This curation improves the clinical applicability of reported results and helps clinicians select appropriate agents across diverse patient populations.
In the ever-evolving landscape of infectious diseases, precision in antimicrobial susceptibility testing (AST) is not just a technical requirement; it’s a cornerstone of effective patient care and public health. Bacterial infections are something general practice veterinarians deal with every day, and we play a major role in the changing resistance dynamics of our world.
Antech is committed to fighting antimicrobial resistance, working to provide faster, more reliable susceptibility information that supports and empowers veterinarians.
By making responsible, evidence-based treatment decisions, we can all work together to combat antimicrobial resistance and make a better world for pets and their humans.
If you’re looking for resources to support antimicrobial stewardship-centered care, Antech offers our customers practical tools, training, and education to enhance clinical communication: Please visit our Microbiology page to learn more.
For questions about specific cases, Antech’s Clinical Microbiologist and board-certified Consultation Services team are here to provide guidance, support, and partnership in care.