At Antech™, our microbiology services are designed to give veterinarians answers they can trust. With advanced diagnostics and expert support, we help you:
- Enable accurate microbial pathogen identification
- Choose the most effective treatment options
- Improve patient outcomes with confidence
We’re also committed to the bigger picture: fighting antimicrobial resistance (AMR). By following the latest CLSI guidelines and European Medical Agency (EMA) published antibiotic prescribing tiers, we support responsible antibiotic use — helping you protect not only your patients but the wider community.
Learn about new MIC panel enhancements and HealthTracks report:
Questions about our MIC Updates?
What’s changing in how Antech reports MICs for dogs and cats?
Recent updates enable Antech to report minimal inhibitory concentrations (MICs) and susceptibility results using most up-to-date veterinary-specific Clinical and Laboratory Standards Institute (CLSI) guidelines rather than human breakpoints. This change aims to support veterinarians in making more accurate treatment decisions and emphasizes responsible antimicrobial use. Additionally, these updated reports are exclusively available in HealthTracks. Not registered for HealthTracks? Click to learn how to register today.
Who is CLSI, and why are they important for veterinary medicine?
The Clinical and Laboratory Standards Institute (CLSI) is an international, non-profit organization that develops consensus-based guidelines for laboratory testing, including microbiology and antimicrobial susceptibility testing (AST). CLSI brings together veterinary and human health experts to set evidence-based standards that ensure lab results are accurate, clinically meaningful, and reproducible across different settings. CLSI publishes veterinary-specific documents (e.g., VET01, VET02, VET06, VET09 in Further Reading) that outline species-specific testing methods, breakpoints, and interpretive criteria for dogs, cats, horses, and more.
What guideline changes has CLSI made for veterinary MIC interpretation?
CLSI has updated its veterinary antimicrobial susceptibility testing guidelines to improve the accuracy and relevance of MIC interpretation for animals. Key changes to the antimicrobial susceptibility testing guidelines include:
- Veterinary-specific breakpoints for species like dogs and cats
- New “Susceptible, Dose-Dependent (SDD)” category for dose-adjusted treatment options
- Revised breakpoints for select drug-pathogen combinations
- Clearer reporting standards to support consistency and clinical decision-making
What are the EMA categories that I see on the report and why are they important?
Antech now provides suggested prescribing tiers for antimicrobials under the categories Avoid, Restrict, Caution, and Prudence to guide selection and support stewardship. These are assigned to the medication based on risk of antimicrobial resistance and importance to human health.
Why did Antech use EMA categories instead of another system?
We chose EMA (European Medicine Agency) categories for their straightforward guidance: their source is less important than their practical role in promoting thoughtful, responsible antimicrobial use for every patient case. (See Interpretation for how to use them.)
Why did Antech remove dose recommendations from reports?
Every patient is unique and safe, effective dosing depends on many factors. These include, for example, kidney and liver function; owner compliance and practicality—along with many others. To help tailor treatment, we encourage you to consult trusted references like Plumb’s
Veterinary Drug Handbook, ISCAID, or ACVIM guidelines, and other pharmacology resources. For especially complex cases, specialist input can be invaluable.
These updates are only available in HealthTracks. Why are MIC reports not updated in AntechOnline?
AntechOnline is our legacy platform and is no longer being enhanced. To best support veterinarians, Antech is investing in HealthTracks—our one digital diagnostic platform designed to deliver continuous innovations, streamlined workflows, and enhanced reporting. HealthTracks provides the comprehensive diagnostic support our customers need, while AntechOnline cannot fully support these growing requirements. For the most up-to-date features and results, we encourage you to use HealthTracks
Our clinic hasn’t used HealthTracks before. How do we access these new MIC reports?
You can log into HealthTracks using the same credentials you use for AntechOnline. If you prefer MIC results to be emailed directly to your hospital’s email address, please contact HealthTracks support at [email protected] to have your email preferences updated.
Please note, fax reports are not yet available with these new features but will be in the near future
I only use my practice management software to view my results and don’t have a login to HealthTracks; How can I view the best report?
Customers that access their results within an integrated PIMS interface will continue to see MICs and S/I/R interpretations in the manner in which their PIMS chooses to show that data; however, PIMS views are dependent on the software company and new Antech-specific features are only available in HealthTracks.
If you prefer MIC results to be emailed directly to your hospital’s email address, please contact HealthTracks support at [email protected] to have your email preferences updated.
Please note: Fax reports are not yet available with these new features but will be in the near future

Listen to our podcast episode on the new MIC updates!
Board-certified veterinary microbiologist and immunologist Meera Surendran Nair (DVM, MS, MSCTR, PhD, DACVM), Director of Infectious Disease at Antech™ and board-certified veterinary microbiologist and immunologist, joins Tails From the Lab to discuss the recent updates to antimicrobial susceptibility reporting and clinical breakpoints. She explains how these changes impact veterinary diagnostics and antimicrobial therapy decisions, helping clinicians select the right antibiotic at the right dose to effectively treat infections while minimizing unnecessary antimicrobial exposure.
At Antech™, Microbiology Means More
We deliver accurate, timely, and actionable microbiological diagnostics that empower veterinarians to make informed treatment decisions—enhancing animal health, reducing antimicrobial resistance, and supporting better clinical outcomes.
What Sets Us Apart
Rapid Turnaround Times
Fast results when they matter most — with positive aerobic and urine culture and susceptibility results reported in just 48-72 hrs.
Integrated Resources
Educational tools, FAQs, and access to our consultation team support you in interpreting results and managing complex cases with confidence.
Clinically Relevant Reports
From pathogen identification to resistance profiles, our new HealthTracks.com reports are clear, actionable, and backed by expert microbial insights.
Antibiotic Stewardship Support
Data-driven recommendations guide targeted therapies, reducing reliance on empirical treatment and promoting responsible antimicrobial use.
Species-Specific Expertise
Whether companion animals, exotics, or equine, our team understands the unique microbiological challenges across species.
Our Commitment
We promise to be your partner in veterinary care. Through scientific rigor and clinical empathy, we empower you to deliver the best possible care — every patient, every time.
From Specimen to Code
Where do I start?
Sending a specimen should be simple. Here’s how we make it easy — from collection to code selection.
General Workflow
- Determine specimen type for submission
- Submit your specimen following our collection guidelines
- Specimen is processed in one of our state-of-the-art labs across the U.S. and Canada.
- Results:
- Presence or absence of bacteria reported in 24 hours
- Culture ID and sensitivity results reported within 2–4 days.
Sample Guidelines & Test Codes
- Urine
- M130 (Canada: CM130) – Culture, urine with FIRSTract™
- Skin
- M020 (Canada: CM020) – Culture, Aerobic
- M040 (Canada: CM040) – Culture, Aerobic/Anaerobic
- M080 (Canada: CM080) – Culture, Fungal
- M240 (Canada: CM240) – Culture, Dermatophytes
- Blood
- M060 (Canada: CM060) – Culture, Blood Aerobic Only
- M061 (Canada: CM061) – Culture, Blood Aerobic/Anaerobic
- M083 (Canada: CM083) – Culture, Blood Fungal
- Ear
- M255 (Canada: CM255) – Aerobic Culture, Ear
Species-Specific Considerations
- Equine: Tailored reporting to reflect equine-specific pathogens and clinical needs.
- Exotics: Custom antimicrobial susceptibility Kirby-Bauer panels to ensure accurate results for avian, reptile, and small mammal patients.
Technology, Speed, and Support
Why veterinarians choose Antech Microbiology
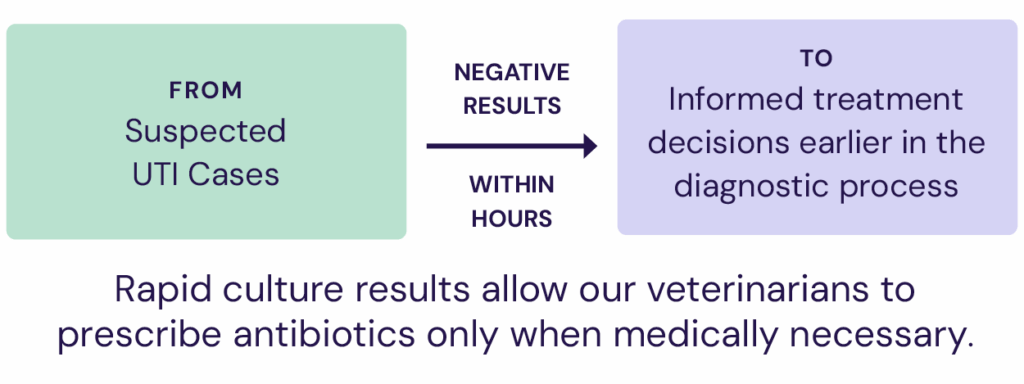
- FIRSTract™ for faster urine culture results — confirms the presence of actively growing bacteria within hours
- Rapid microbial identification using mass spectrometry to detect each organism’s unique protein ‘fingerprint’ (MALDI-TOF)
- Guideline-driven: Clinical and Laboratory Standards Institute (CLSI) for accurate microbial identification and antimicrobial susceptibility and European Medicines Agency (EMA) antibiotic categorization to promote responsible antimicrobial use.
- Expert consultation: Access to internal medicine specialists and a board-certified veterinary microbiologist.
- Fast turnaround: Initial results within 24 hours and culture ID with antimicrobial susceptibility results reported in 2 – 4 days.

Meet our Microbiologist
Dr. Meera Surendran Nair, DVM, MS, MSCTR, Ph.D. DACVM is the Director, Infectious Disease for Antech Diagnostics™. She is a Board-Certified Veterinary Microbiologist and Immunologist. Dr. Surendran-Nair is passionate about diagnostic innovations & collaborates to deploy diagnostic solutions unraveling diagnostic innovations disease riddles. She nurtures diagnostic and antimicrobial stewardship.
Resources
Your go-to tools for confident microbiology testing.
Flyers & Downloads
- Blood Culture Guide (U.S.)
- Blood Culture Guide (Canada)
- Ear Culture Guide (U.S.)
- Ear Culture Guide (Canada)
- Microbiology Services Detailer
- Microbiology Sample Collection & Handling Guide (By Source) (U.S.)
- Microbiology Sample Collection & Handling Guide (By Source) (Canada)
- Skin Culture Guide (U.S.)
- Skin Culture Guide (Canada)
Contact & Support
Need help choosing the right test code or interpreting your report? We’re here to help.
- Consultation Support: Speak with our clinical experts. (US) 800-872-1001, Option 2; (CA) 800-341-3440, Option 2
- Customer Service: For logistics, ordering, and submission questions. (US) 800-872-1002 Option 0; (CA) 800-341-3440, Option 0
Experience precise, accurate, flexible microbiology solutions for superior care.
Webinars
Update on the changes in CSLI Breakpoints
MIC Overview
A Review of the Approach to a Culture and Sensitivity Profile Interpretation
What’s new at Antech
-

Mental Health in Veterinary Medicine Part Six: Creating a Culture of Wellbeing and Becoming an Agent of Change with Dr. Jen Brandt
This excerpt is from the final episode in a three-part podcast series on mental health in veterinary medicine from the Tails From the Lab podcast, hosted by Brad Ryan (MSC, DVM, MPH), Senior Professional Services Veterinarian at Antech, in conversation with Jen Brandt (LISW-S, PhD), Director of Member Wellbeing Initiatives at the AVMA. In this episode, Drs. Brad and Jen talk about how overworking…
-

Mental Health in Veterinary Medicine Part Five: Creating a Culture of Wellbeing with Dr. Jen Brandt
This excerpt is from the final episode in a three-part podcast series on mental health in veterinary medicine from the Tails From the Lab podcast, hosted by Brad Ryan (MSC, DVM, MPH), Senior Professional Services Veterinarian at Antech, in conversation with Jen Brandt (LISW-S, PhD), Director of Member Wellbeing Initiatives at the AVMA. In this episode, Drs. Brad and Jen talk about how to…
-

Mental Health in Veterinary Medicine Part Four: Self-Compassion and Perfectionism with Dr. Jen Brandt
This is the second episode in a three-part series on mental health in veterinary medicine from the Tails from the Lab podcast, hosted by Brad Ryan (MSC, DVM, MPH), Senior Professional Services Veterinarian at Antech, in conversation with Jen Brandt (LISW-S, PhD), Director of Member Wellbeing Initiatives at the AVMA. In this episode, Drs. Brad…