Antech™ Expands trūRapid™ FOUR and trūRapid™ FIV/FeLV Rapid Tests to Canada After CFIA Approval
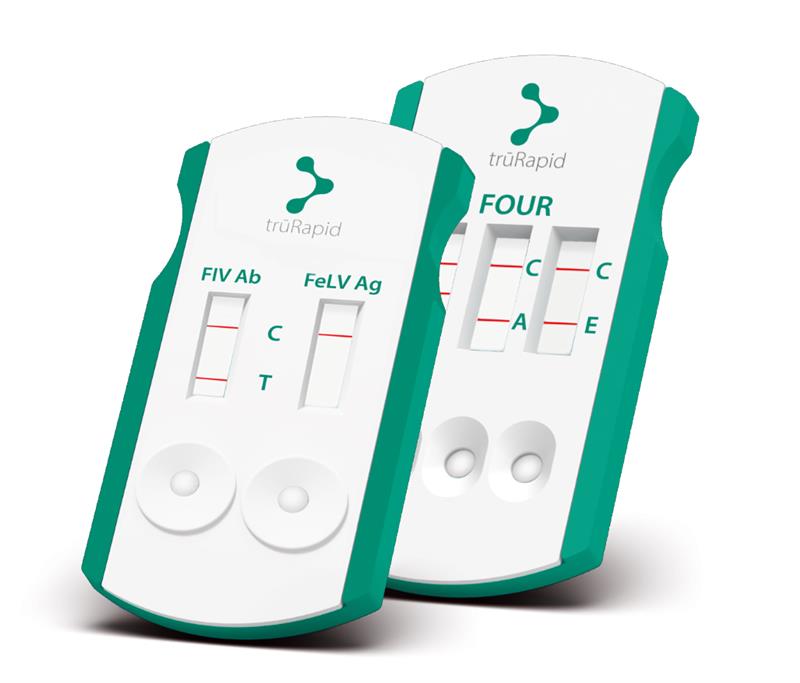
Now available in Canada:
trūRapid™ FOUR: simple, fast, and accurate screening for canine vector-borne diseases
trūRapid™ FIV/FeLV: Feline leukemia (FeLV) and immunodeficiency virus (FIV)
LOVELAND, Colo.–(BUSINESS WIRE)–Antech™, a global veterinary diagnostics company, today announced the Canadian Food Inspection Agency (CFIA) has granted regulatory approval for trūRapid™ FOUR, a comprehensive in-house canine vector-borne disease screening test, and trūRapid™ FIV/FeLV, a two-in-one infectious disease test that simultaneously screens for Feline Immunodeficiency Virus (FIV) antibodies and Feline Leukemia Virus (FeLV) antigen. These rapid lateral-flow tests provide in-house screening for veterinary clinics and animal care facilities, supporting early detection using whole blood, serum, or plasma.
trūRapid™ FOUR detects:
- Canine antibodies to Anaplasma spp., Ehrlichia spp., and Lyme C6 (Borrelia burgdorferi)
- Heartworm (Dirofilaria immitis) antigen
trūRapid™ FIV/FeLV detects:
- FeLV antigen and FIV antibodies – early detection of these viruses is an important factor for monitoring and treating illnesses
Jimmy Barr, DVM, DACVECC, Chief Medical Officer for Mars Petcare’s Science & Diagnostics division and Antech, said: “With the commercial availability of the trūRapid™ product line now extended to Canada, we are excited to build on the strong progress and adoption we have already seen in the United States. These tests offer veterinary professionals a fast, reliable, and streamlined solution for infectious disease screening, helping to improve animal health outcomes while simplifying clinic workflows.”
Key Features of trūRapid™ tests include:
- Streamlined Workflow: Requires just a few drops of sample, minimizing the need for additional blood draws or sample centrifugation. Eliminates manual snapping; the test reaction begins automatically when the sample and buffer is directly added.
- Proven Reliability: Excellent sensitivity and specificity when compared to reference methods.
- No Refrigeration: Tests can be stored at room temperature for their entire shelf life, saving refrigerator space, simplifying inventory management, and eliminating the need to warm up the test before use.
- Better Design: Generates less plastic waste with no need for additional sample handling tubes — just the cartridge and a plastic pipette.